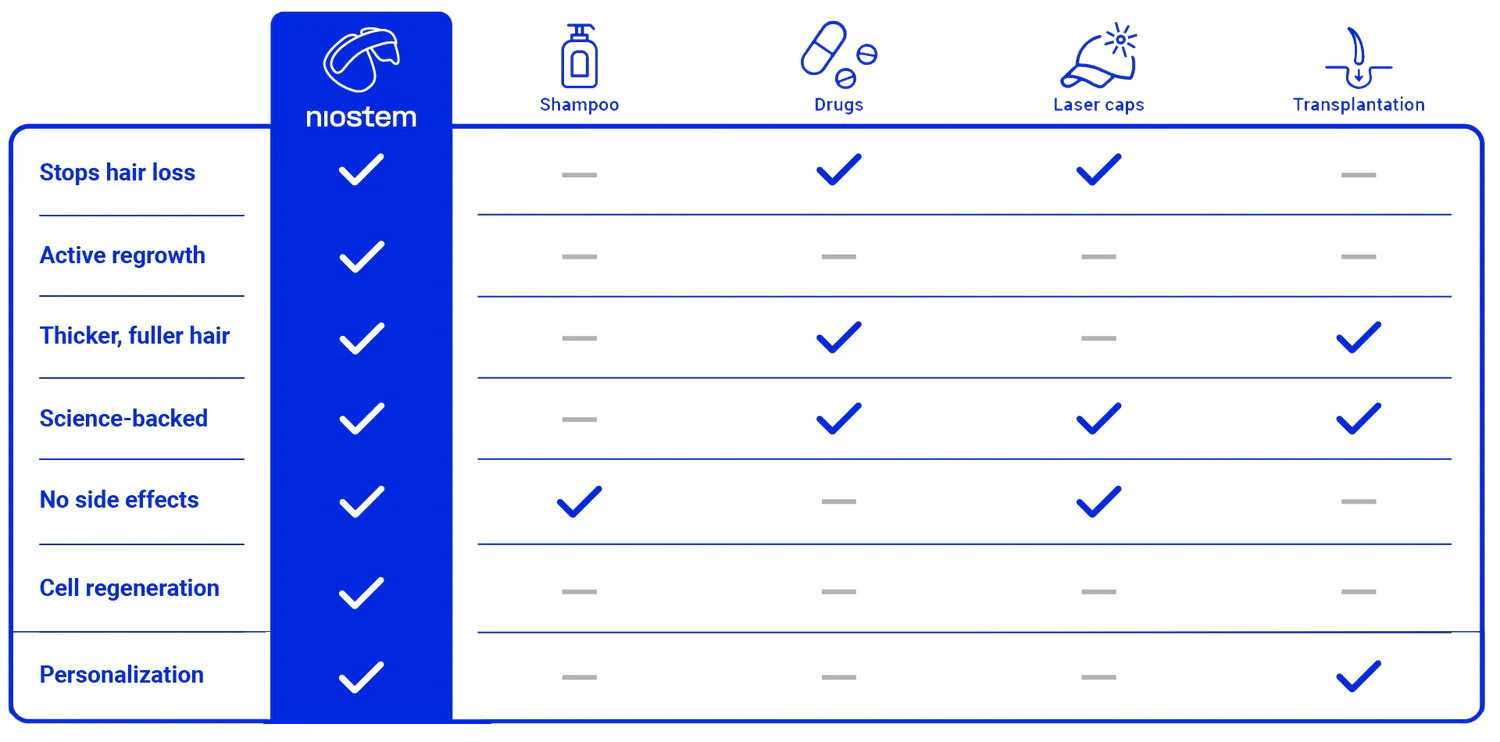
Summary of the results of our latest 6-month clinical trial.
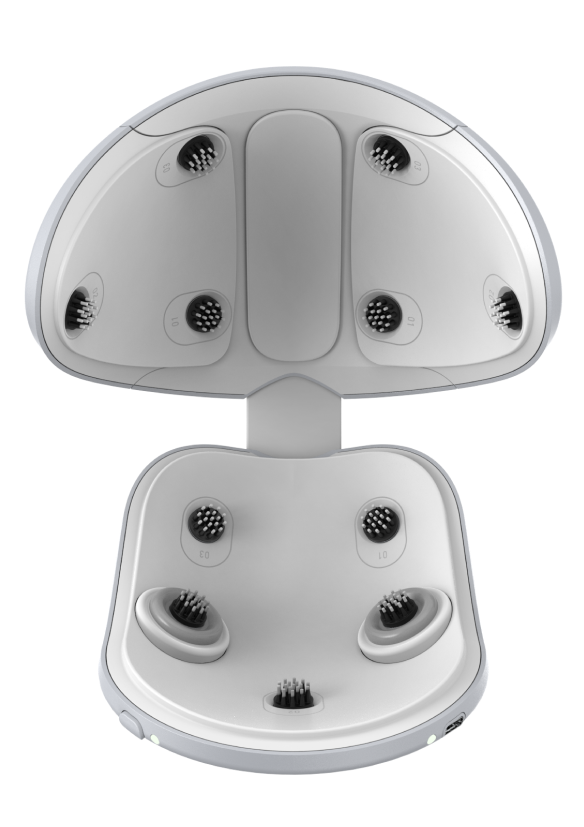
App-controlled, wireless and easy to use – whenever and wherever you want. And at least just 5 days a week.

TrustScore 4.2 | 58 Bewertungen

Groundbreaking research reveals the hidden hair crisis: Dihydrotestosterone (DHT) as the main cause of hair loss.
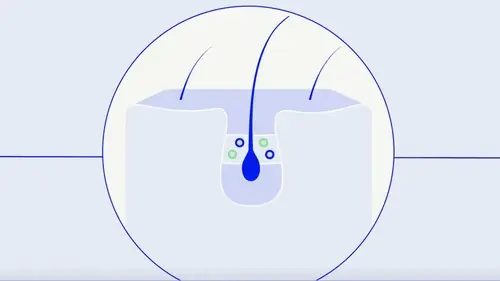
Leading scientists reveal how DHT maintains a ‘stranglehold’ on hair follicles and blocks the hair’s natural growth cycle. Over time, hair becomes finer and smaller until it eventually falls out completely.
The hair is literally starved, which leads to this:
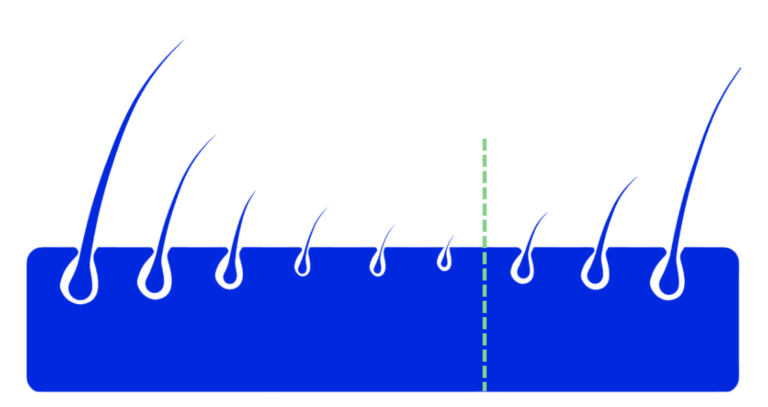
Shrinkage and growth process of the hair root
The niostem wearable revolutionizes the treatment of hair loss.
Instead of relying on influencing DHT like many pharmaceutical products, which often leads to undesirable side effects, niostem uses an innovative alternative: bioelectric stimulation.
This method activates the stem cells in the hair root, restoring the disturbed growth process of the hair follicle.
The result: the hair is put into growth mode, hair loss is not only stopped, but reversed.

*Deutsches Schaubild folgt in Kürze.

Hair stem cells on the scalp are put back into action mode.
Reactivates the growth of your hair not only superficially.
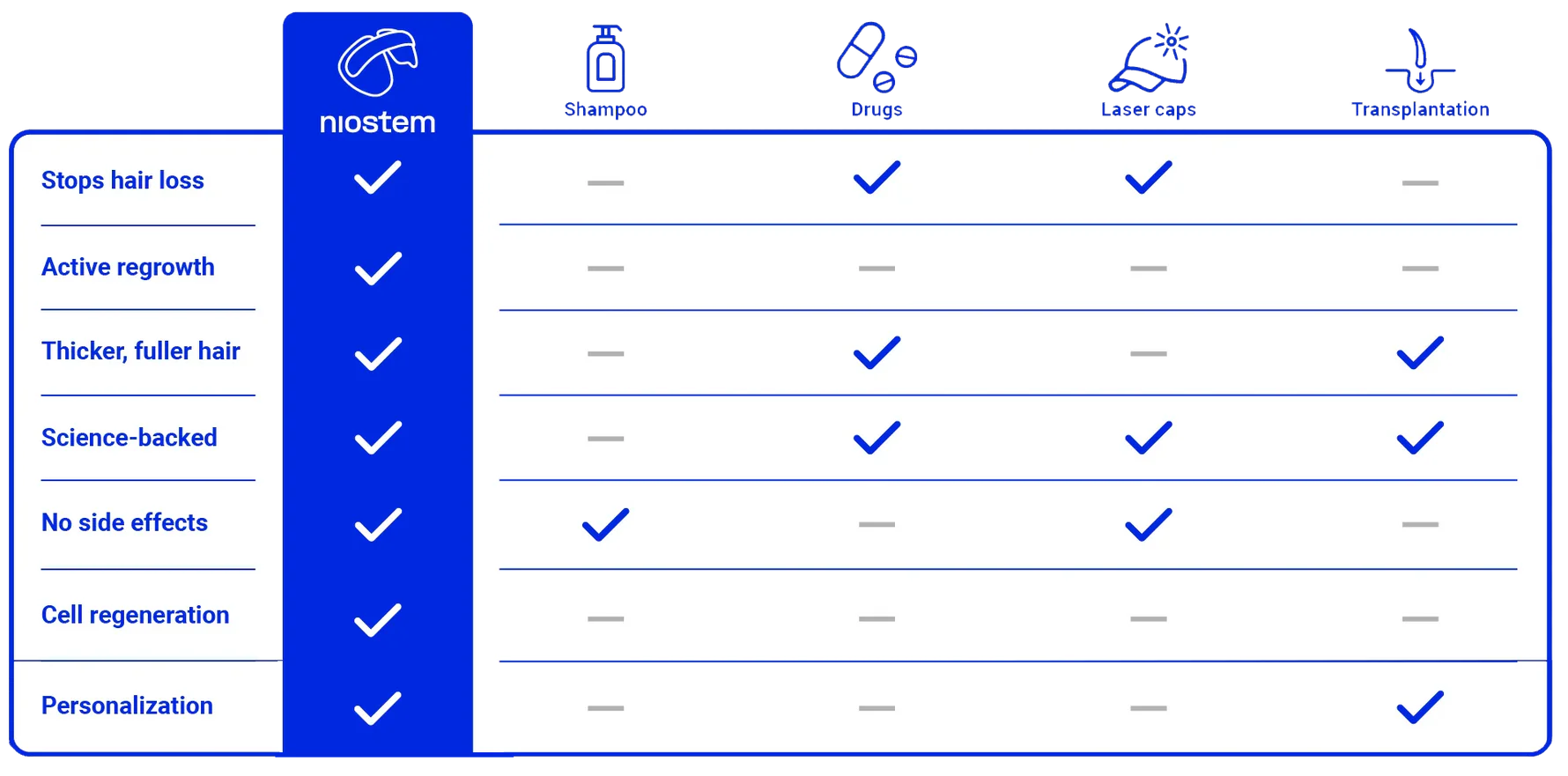
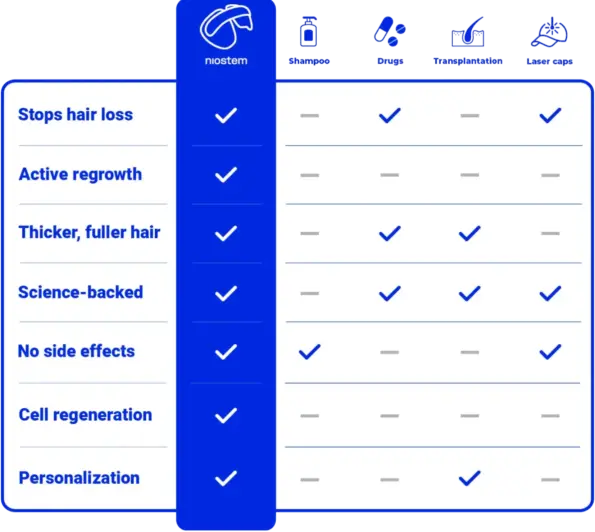
Bio-electrical stimulation without unpleasant sensations.
No interference in the hormone balance.
Safe and targeted against the causes of hair loss.

Bio-electrical stimulation without unpleasant sensations.
Safe and targeted against the causes of hair loss.
No interference in the hormone balance.
You can keep waiting a while for miracles. You can rely on research right now. niostem gets your hair stem cells working again – with unique stem cell reactivation technology. The result: stronger, healthier and thicker hair.







Real results or money back – more info

1.290€*
Free Shipping

Payment in installments with Klarna or PayPal 12 installments
*Limited time offer ; VAT included.
Because we understand that you want to see results.
There are three things you need to do to take advantage of the guarantee:
The guarantee only applies to hereditary hair loss (androgenetic alopecia) in an early to medium stage (Norwood 1-5).
Not suitable if you are affected by e.g:
If you’re not sure whether niostem is right for your situation, write us – we’ll be happy to help: support@niostem.com
We believe in our technology. That’s why we don’t just give you a product – we give you security and transparency. You don’t bear the risk.
niostem’s technology focuses on reawakening the dormant hair stem cells, thus targeting the core problem of male pattern baldness. Rather than employing light in our technology, we physically stimulate the stem cell through a gentle mechanical process. By doing this, we encourage the hair stem cells to convert into the progenitor stage, effectively kickstarting the regrowth process at a cellular level resulting in both thicker and more hairs growing. No drugs. No serious side effects.
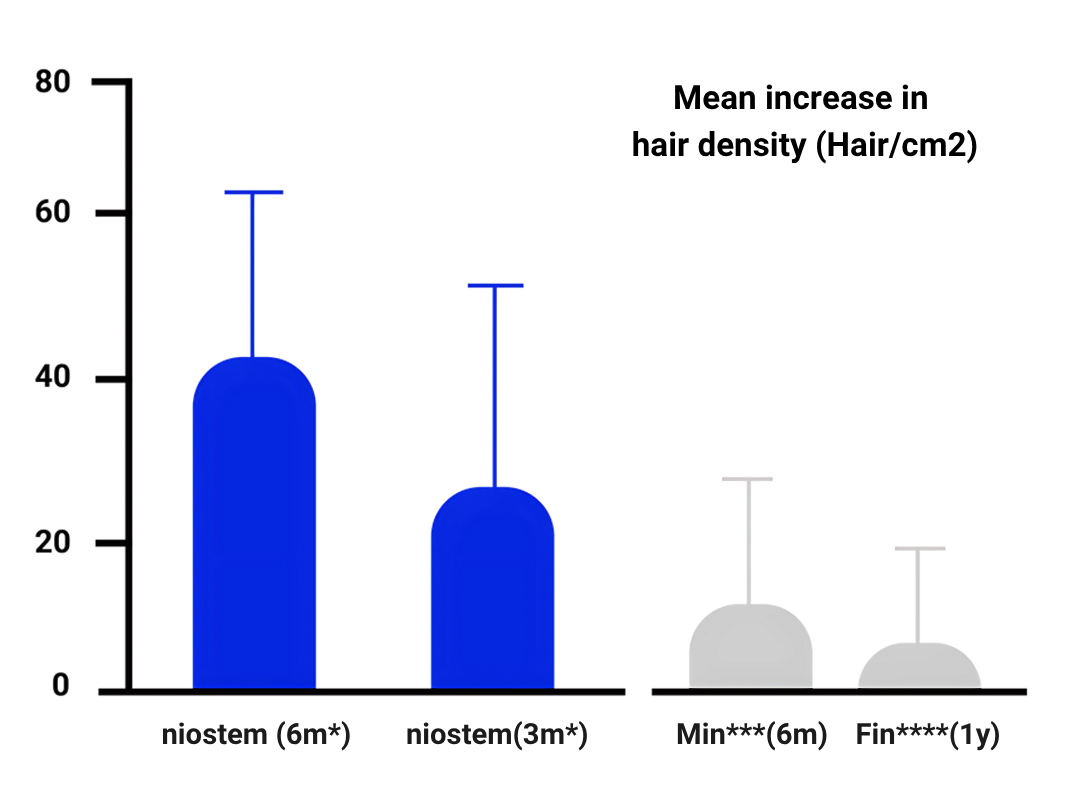
Based on niostem pilot results, 95.4% of participants stopped their hair loss after 3 months 86.3% of them showed hair regrowth with an increase in hair shaft density (mean increase of 12% compared to baseline).
That means, during the first 12 weeks most people can expect to stop hair loss and visually see less fallen hair on the pillow, blankets, bathroom sink, etc.
After 12 – 24 weeks, most people see by themselves the first signs of regrowth in both hair density and hair thickness.
Beyond 24 weeks: 100% of participants stopped their hair loss, and 100% showed hair regrowth. The increase in hair shaft density (19.3% mean increase) and 8.8% increase in hair thickness.
The niostem device uses low-level electrical stimulation delivered through our unique wearable device to kick-start the hair stem cells back into progenitor cells that fuel the hair follicles and hair growth.
Our intelligent system ensures that an invisible network spreads across the upper scalp, generally affected by common hair loss.
Hair loss can be stopped in the first three months and hair growth can be stimulated in the first six months. However, the time it takes to see results depends on many different factors, such as the degree of hair loss, health status, age, hair type, diet, or other underlying medical conditions.
However, you can expect to see better results in terms of regrowth and regeneration in bald areas where your hair follicles were previously reduced in size.
Bioelectrical stimulation has been used in different applications since the 90s (neuromuscular, ophthalmological, nerve and other stimulation fields). To date no adverse effects have been reported.
Additionally, our device complies with European health, safety, and environmental protection standards(CE).
You can compare it to going to the gym. If you go often you will build muscles. But, if you stop going your muscles will slowly fade again. However, if you miss 1 day it is not a disaster.
We recommend a daily application of 30 minutes, but will continue to improve and test our product to recommend other (lower) application frequencies in the future.
In an in-house study, the niostem device was proven more effective when used alone. Therefore, combining solutions could be unnecessary because of the way it works through stem cell reactivation technology, as it’s not essential to block the effects of DHT or increase blood flow to the hair follicle.
On the other hand, it is well known in the hair loss market that combinations of approaches can have additive effects.
niostem has no data to date on combinations with the device.
We recommend you discuss any prescription medicine with your doctor for your specific situation.
Minimal adverse effects included an itchy scalp (scalp pruritus; 9%) and slight headache in the first 2-4 days after the initial use (4.5%), which are also common with approved drugs and laser caps (Adil et al. 2017). All these effects disappeared after a week of use.
Yes, there is!
The niostem wearable has been successfully tested for safety and efficacy in men and women in two independent 6-month clinical trials conducted in 2021* and 2024** in the UK and Germany.
*niostem clinical pilot study. Jellard, Moore & Chacón-Martínez et al. 2025. Journal of Cosmetic Dermatology.**niostem randomized controlled clinical study. Chacón-Martínez et al. Unpublished
The legal warranty covers any hardware defects presumed to have existed at the time of delivery.
Erfahre in einem persönlichen Gespräch, wie du dein Haarwachstum optimal unterstützen kannst – individuell & unverbindlich!
Targeted against hereditary hair loss
niostem is not suitable for people with other types of hair loss, including, but not limited to, cicatricial alopecia, alopecia areata (autoimmune reaction), therapy-induced hair loss, etc.
Check the stage of your hair loss
Make sure that your hair loss is in the range of level 1 to 5 on the Norwood scale (for men) or type I to IV on the Ludwig scale (for women).
Unfortunately, we cannot recommend the use of niostem for advanced stages beyond this.
In these stages, there are usually still enough active hair follicles and stem cells that can be reactivated in a targeted manner using our technology.
At Norwood stage 6, the majority of follicles may already be in a deep dormant phase, making reactivation more difficult.
We recommend an individual assessment in this case – also in consultation with a doctor.
At Norwood stage 7, we currently advise against using our wearable, as the chances of success at this advanced stage are very low according to current knowledge.

For women with hereditary hair loss (female pattern baldness), the clinical data on niostem is promising but not yet conclusive enough to recommend unrestricted use of the wearable. However, this type of hair loss behaves similarly to male hereditary hair loss, which could indicate comparable effectiveness:
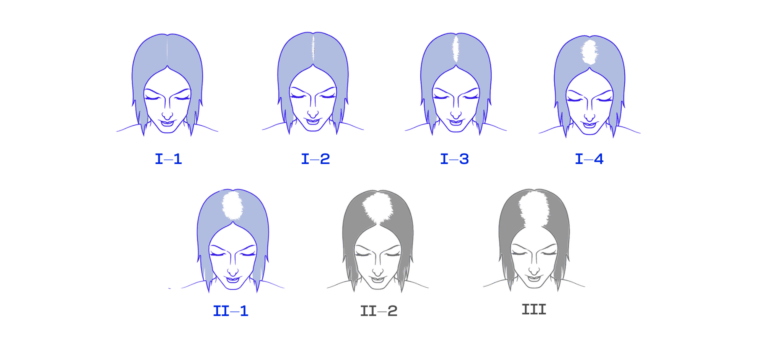
For women: Ludwig types I-2 to II-1
In these stages, there are usually still enough active hair follicles and stem cells present that can be specifically reactivated by our technology. Here, we recommend an individual assessment – ideally with medical consultation.
At Ludwig type II-2, a large proportion of follicles may already be in a deep resting phase, making reactivation more difficult. Here, too, we recommend an individual assessment – ideally with medical consultation.
At Ludwig type III, with severe thinning in the crown area, we currently advise against the use of our wearable, as the chances of success at this very advanced stage are, according to current knowledge, very low.
UNTERSTÜTZUNG
RECHTLICHES
MEHR niostem
Haftungsausschluss: Dieses Produkt ist nicht zur Diagnose, Behandlung, Heilung oder Vorbeugung von Krankheiten bestimmt und die Ergebnisse können variieren. Diese Informationen stellen keinen medizinischen Rat dar und sollten nicht als solche ausgelegt werden. Konsultieren Sie Ihren Arzt, bevor Sie Ihr reguläres, ärztlich verordnetes Behandlungsschema ändern.
Lokale Zölle und Einfuhrsteuern sind nicht im Preis enthalten und liegen in der Verantwortung des Kunden. Der Versand erfolgt aus Deutschland.

„Das niostem Wearable wurde in Deutschland am Max-Planck-Institut von Dr. Carlos Chacón-Martínez entwickelt – und wird in Köln weitergeführt“

Es gilt unsere Datenschutzrichtlinie.

Buche unkompliziert einen Termin für ein persönliches Beratungsgespräch.
Es gelten unsere Datenschutzrichtlinie und Nutzungsbedingungen
Du wirst nun auf unsere offizielle Homepage (englisch) zum Kaufabschluss weitergeleitet.
Schieben Sie den Regler auf den Bildern nach rechts und links,
um den Vorher-Nachher-Effekt zu sehen.